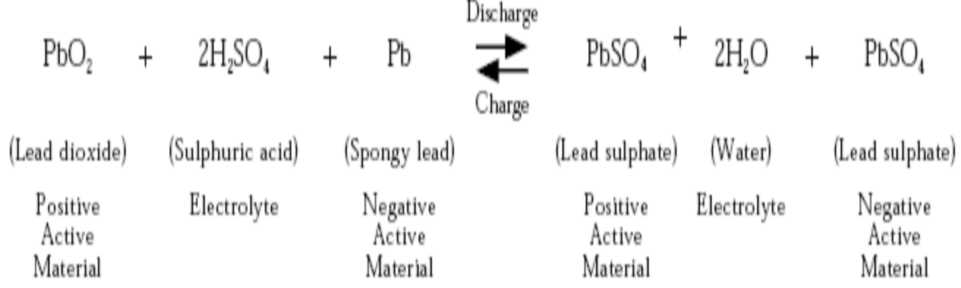
Theory of the lead-acid storage battery
Theory of the lead-acid storage battery
What is VRLA battery?
Reaction at positive plate (oxygen generation)
2H2O =O2 + 4H+ + 4e-
Reaction at negative plate
(chemical reaction of spongy lead with oxygen)
2Pb + O2= 2PbO
(chemical reaction of PbO with electrolyte)
2PbO + 2H2SO4=2PbSO4+ 2H2O
(Reduction of PbSO4 )
2PbSO4 + 4H+ + 4e- =2Pb + 2H2SO4
Total reaction at negative plate
O2 + 4H+ 4e-= 2H2O
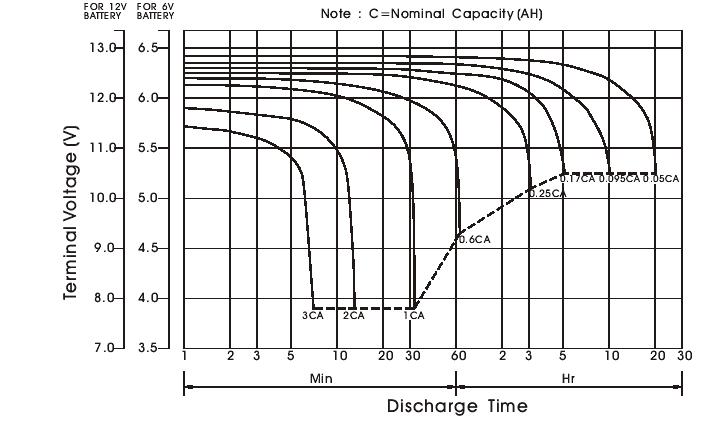
Discharge characteristics
- Discharge curves
-Deep discharge
-what is deep discharge
-deep discharges are critical for both electrodes:
Positive
Negative
Dendrites
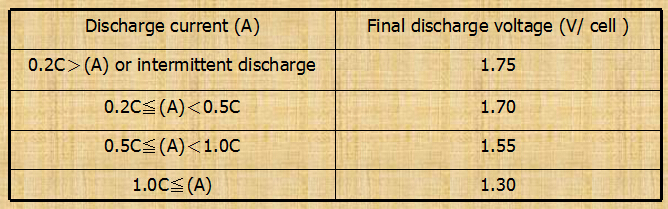
-Battery discharge current vs. discharge voltage
-Cycle life in relation to depth of discharge
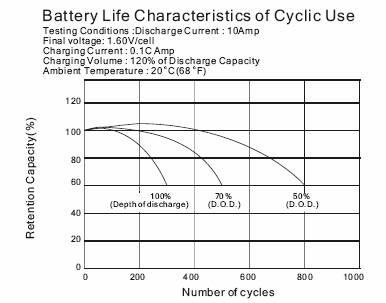
After over discharged, the batteries should be recharged as soon as possible by correct ways.
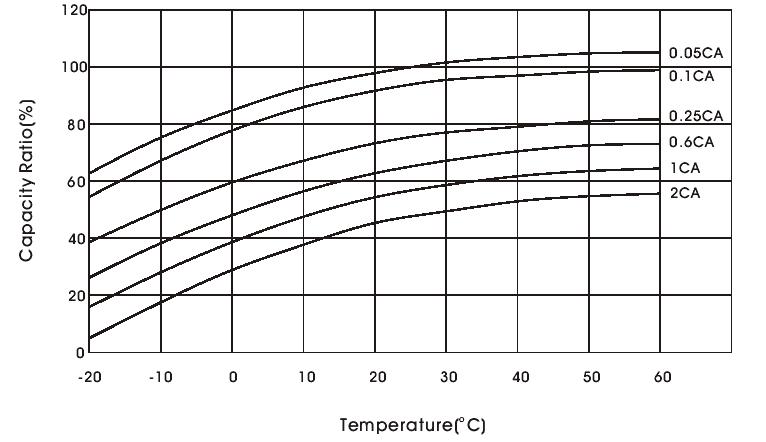
-Temperature effect on battery capacity
-Battery self discharge characteristics
- short circuit
- impurity
- inherent reaction at negative electrode
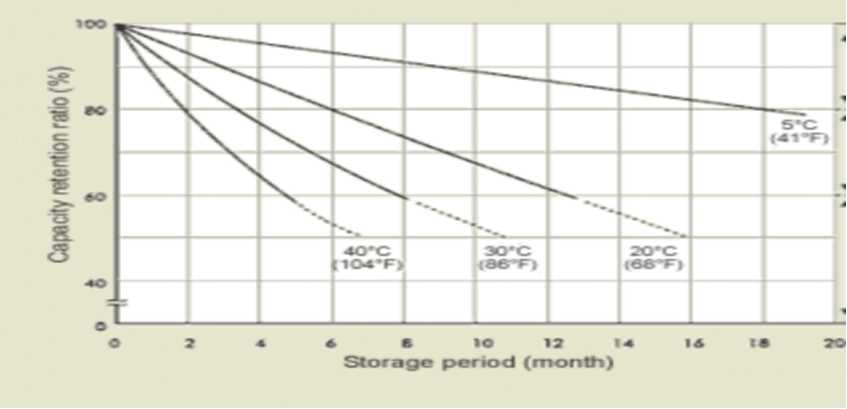
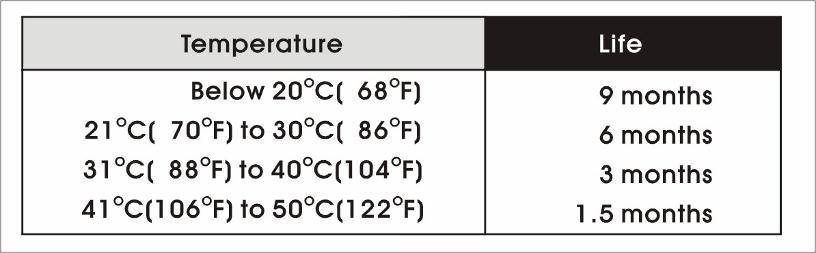
-Shelf life
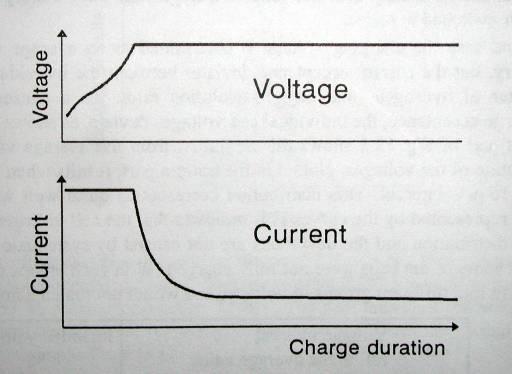
Charge characteristics
Constant-voltage charging/Floating charging:
2.25-2.30V/cell for standby, 2.40-2.50V/cell for cycle use with the initial current is controlled at 0.1CA
Constant current charging
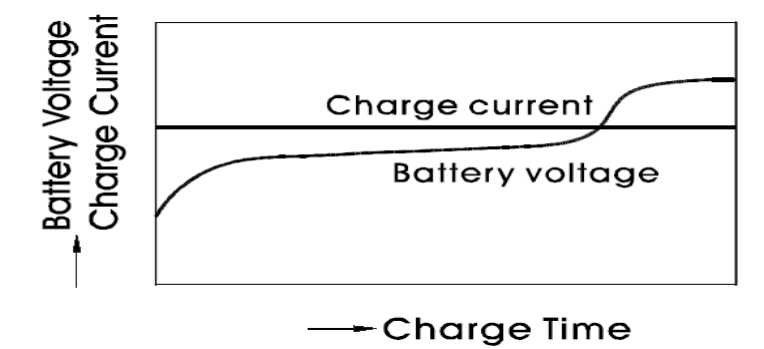
Two stage constant voltage charging
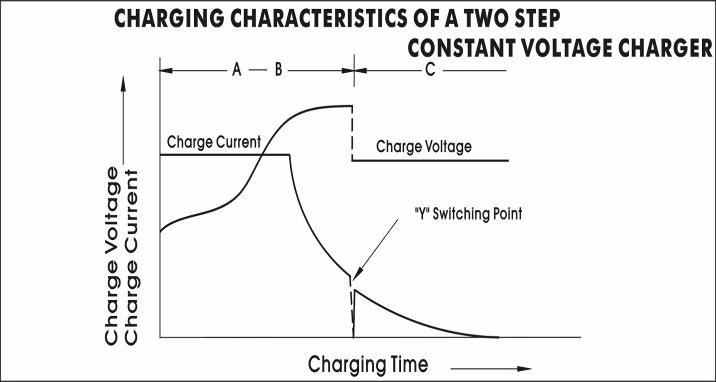
Initial charge current ------ 0.3C.A (max.)
1st stage voltage – 2.45/cell (2.40 to 2.50 Volts /cell)
2nd stage voltage------2.28v/cell (2.25 to 2.30 volts/cell)
Switching current from---0.05C (0.04C to 0.08C.A)
Recharge stored battery
|
Battery age |
Top charging recommendations |
|
Within 6 months after manufacture |
4 to 6 hours at constant current of 0.1C Amps or 15 to 20 hours at constant voltage of 2.40 vpc |
|
Within 12 months after manufacture |
8 - 10 hours at constant current of 0.1C Amps or 20 to 24 hours at constant voltage of 2.40 vpc |
know more about:
Bullspower lead-acid storage battery structure













