How To Select The Right Battery For Your Application?
|
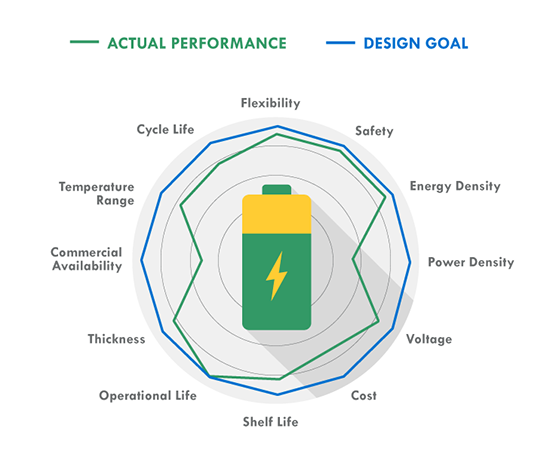
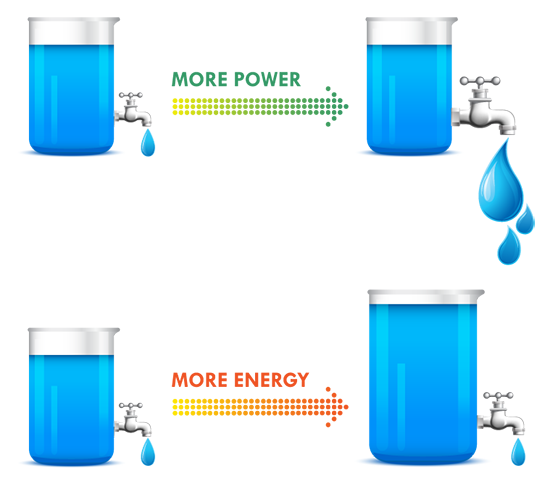
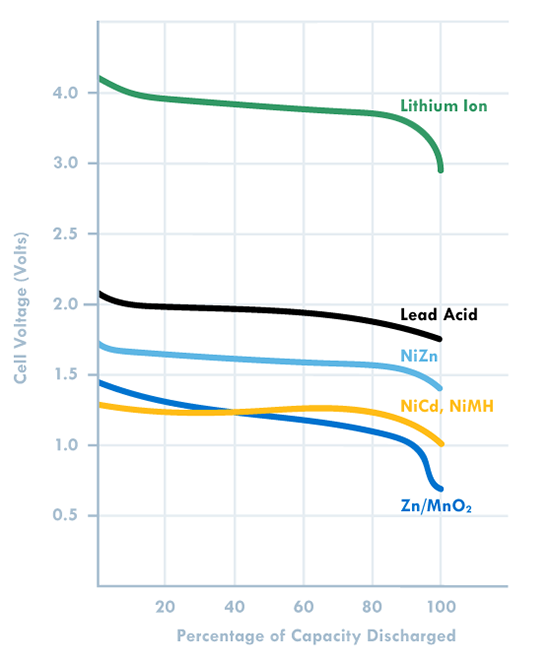
As more systems go mobile and remote, batteries are becoming increasingly important to design. Make sure you choose the right one by keeping these ten design factors in mind, then use our battery chemistry comparison chart to determine if Lead Acid, Alkaline, Nickel-Metal Hydride or Lithium Ion is best for your build. The one thing to remember about battery selection is that there is no such thing as a perfect battery that works for every application. Selecting the right battery for your application is about identifying the most important battery metrics and trading these off against others. For instance, if you need a lot of power for your application, cell internal resistance needs to be minimized, and this is often done by increasing electrode surface area. But this also increases inactive components such as current collectors and conductive aid, so energy density is traded off to gain power. A lead acid battery works great in an automotive starter battery where it provides the required high rate capability. However, with its toxicity and low energy density it would be a terrible choice for a portable electronics application. 1. Energy Density: Energy density is determined by comparing the potential energy available to the overall weight of the system. This means that if your application has high energy density, your application has high potential energy for a low amount of weight. Conversely, low energy density means your battery will be heavy and have less available power. A good analogy for power versus energy is to think of a bucket with a spout. A larger bucket can hold more water and is akin to a battery with high energy. The opening or spout size from which the water leaves the bucket is akin to power – the higher the power, the higher the drain rate. To increase energy, you would typically increase the battery size (for a given chemistry), but to increase power you decrease internal resistance. Cell construction plays a huge part in obtaining batteries with high power density. 2. Current Availability (Power Density): When a device has high power density it means it can sustain high current draws for a ‘large’ period of time (more than a few milliseconds). 3. Durability: Physical external factors can greatly affect the performance of your battery. Different battery chemistries are more susceptible than others to factors such as impact, temperature, humidity, vibrations, magnetic fields, etc. 4. Lifetime: There are two main factors to a battery’s lifetime: charge life and total life. Charge life is the amount of time that potential energy will stay in the battery without trickling out. Total life is the number of charge cycles that a battery will support. 5. Battery Memory: Batteries are susceptible to being ‘trained’ (for lack of a better term) to hold less than their total available charge. For example, if you use a Nickel-Metal Hydride battery from a full charge down to 40% of its full charge capacity repeatedly, it will eventually only be able to hold 60% of its original intended charge capacity. 6. Shelf life – This refers to how long a battery will sit in a storeroom or on a shelf before it is used. Primary batteries have much longer shelf lives than secondary. However, shelf life is generally more important for primary batteries because secondary batteries have the ability to be recharged. An exception is when recharging is not practical. 7. Chemistry – Many of the properties listed above are dictated by cell chemistry. We will discuss commonly available battery chemistries in the next part of this blog series. 8. Physical size and shape – Batteries are typically available in the following size formats: button/coin cells, cylindrical cells, prismatic cells, and pouch cells (most of them in standardized formats). 9. Cost – There are times when you may need to pass up a battery with better performance characteristics because the application is very cost sensitive. This is especially true for high volume disposable applications. 10. Transportation, disposal regulations – Transportation of lithium based batteries is regulated. Disposal of certain battery chemistries is also regulated. This may be a consideration for high volume applications. There are many considerations when selecting a battery. Several of these are related to chemistry, while others are related to battery design and construction. This makes it harder and sometimes meaningless to do a battery metric comparison without a more fundamental understanding of the factors that affect that metric, a topic we’ll explore in the second blog of this series. |